For more than 35 years, healthcare providers and people with rare bleeding disordersa have trusted NovoSeven® RT. It’s the first recombinant factor VIIa (rFVIIa) that is:
Experience where it matters.
Indicated for people of all ages with hemophilia A or B with inhibitors, congenital FVII deficiency, and Glanzmann's thrombasthenia when platelets don’t work, and adults with acquired hemophilia.
Approved for use before, during, and after surgery and procedures
The only rFVIIa used for breakthrough bleeds in the Hemlibra® pivotal clinical trials
aCompassionate use, also known as expanded access, began enrolling in 1988; FDA approval received in 1999 for people with hemophilia A or B with inhibitors.
Krystal and her son Dallas, who lives with
Glanzmann’s thrombasthenia (GT) with resistance
to platelets and uses NovoSeven® RT.
Experience where it matters.
For more than 35 years, healthcare providers and people with rare bleeding disordersa have trusted NovoSeven® RT. It’s the first recombinant factor VIIa (rFVIIa) that is:
- Indicated for people of all ages with hemophilia A or B with inhibitors, congenital FVII deficiency, and Glanzmann's thrombasthenia when platelets don’t work, and adults with acquired hemophilia.
- Approved for use before, during, and after surgery and procedures
- The only rFVIIa used for breakthrough bleeds in the Hemlibra® pivotal clinical trials
aCompassionate use, also known as expanded access, began enrolling in 1988; FDA approval received in 1999 for people with hemophilia A or B with inhibitors.
Krystal and her son Dallas, who lives with Glanzmann’s thrombasthenia (GT) with resistance to platelets and uses NovoSeven® RT.
Is NovoSeven® RT right for you?
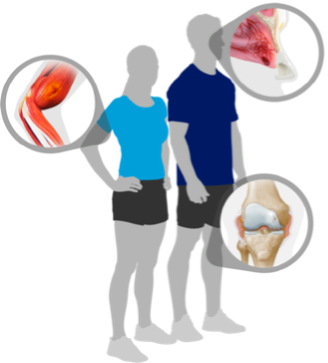
See how NovoSeven® RT can help you control bleeds related to:
![Syringe and vials of NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/cta/CTA-safety-profile.png)
Safety profile with proven experience.
NovoSeven® RT is designed with your safety in mind. It’s the only rFVIIa used for breakthrough bleeds in the Hemlibra pivotal clinical trials.
Studies also show it has a low rate of thrombotic adverse events (blood clots). The most common and serious side effects are blood clots.

Your treatment, your way.
Your treatment should accommodate your lifestyle—take it from people who choose NovoSeven® RT. Hear from others with rare bleeding disorders to learn what makes this treatment the right choice for them.

How can we help?
Novo Nordisk Rare Blood Community Liaisons (RBCLs) are here to answer your questions about NovoSeven® RT and connect you with resources to help you manage your bleeding disorder.
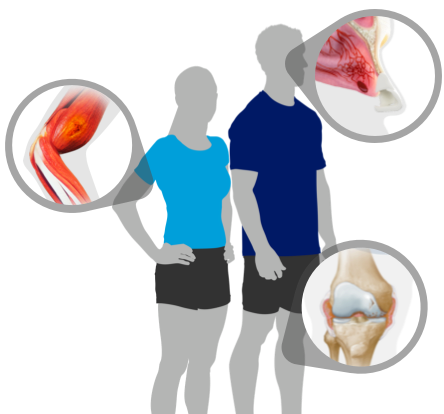
Stay prepared for bleeds
Did you know that identifying bleeds quickly is important? Learn the signs and symptoms of bleeds so you can identify them when and where they happen.
New to NovoSeven® RT?
Here’s how to get started:
I have just been prescribed NovoSeven® RT.
I am considering
NovoSeven® RT.
New to NovoSeven® RT? Here’s how to get started:
I have just been prescribed NovoSeven® RT.
I am considering NovoSeven® RT.
![NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/global/logo-novoseven-rt.png)
