Administration, step by step.
You’ll be administering your factor by intravenous injection, either at a Hemophilia Treatment Center, a health care provider’s office, or at home, after you’ve received training.
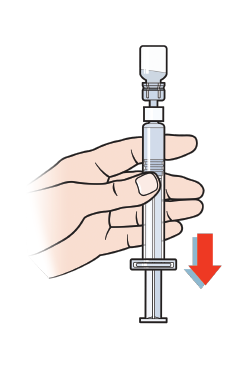
First, invert the NovoSeven® RT vial and slowly draw the solution into the syringe.
Next, detach the syringe from the vial adapter by turning the syringe counterclockwise.
Attach the syringe to the luer end of an infusion needle set.
Then, inject the reconstituted NovoSeven® RT intravenously slowly over 2 to 5 minutes.
When you’re finished, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused NovoSeven® RT, and other waste materials.
![NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/global/logo-novoseven-rt.png)