NovoSeven® RT is proven effective to treat factor VII deficiency (FVIId) at home and is 93% effective at stopping nonsurgical and surgical bleeds in people with FVIId.a
Controls bleeds, wherever they happen.
aFrom an analysis of published cases and people who took part in trials and registries.
Effectively control bleeds.
90% efficacy in bleed control in trial patients.
Established safety profile that comes from experience.b
NovoSeven® RT is a recombinant product, meaning it is made without human blood or plasma, which minimizes the possibility of viral contamination. Serious blood clots that form in veins and arteries with the use of NovoSeven® RT have been reported.
bCompassionate use, also known as expanded access, began enrolling in 1988; FDA approval received in 1999.

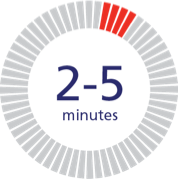
Fast to infuse.
NovoSeven® RT provides low infusion volume, so infusing takes 2 to 5 minutes.c NovoSeven® RT is given as an intravenous (IV) bolus injection.
cAdminister as a slow bolus injection over 2 to 5 minutes, depending on the dose administered.
NovoSeven® RT (90 mcg/kg)
Meet Taylor.
NovoSeven® RT fits in with what she likes to do.
Portability that fits an active lifestyle.
NovoSeven® RT is room temperature stable up to 77 °F so you can be on the go.d And, with compact packaging, you can take it along where you need to go.
dNovoSeven® RT should be stored between 36 °F and 77 °F.
For complete storage information, please see Prescribing Information.

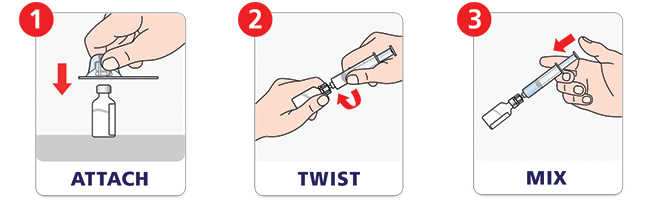
Quick to mix.e
NovoSeven® RT with MixPro® makes mixing a dose as easy as attach, twist, and mix. The prefilled syringe saves time—there are no extra steps to fill a syringe with diluent.
After mixing, NovoSeven® RT may be stored at room temperature in the vial for up to 3 hours.f
eCompared with using histidine vials.
fFor complete storage and handling instructions, please see Prescribing Information.
What if I need surgery or a procedure?
We understand that you have concerns about controlling bleeds if you ever need surgery or a procedure. The good news is that NovoSeven® RT prevents bleeds during and after surgery and procedures in people with FVIId.
Talk to your doctor for more information on the surgical use of NovoSeven® RT.
About factor VII deficiency (FVIId).

What is FVIId?

FVIId is a rare bleeding disorder in which there are low levels of factor VII in the blood. Factor VII plays an important role in the blood-clotting process. When there is not enough factor VII in the blood, clotting can take much longer than normal or may not occur at all.
- Mild to moderate FVIId is marked by increased bleeding after surgery and trauma
- Severe FVIId is marked by spontaneous, severe, and even life-threatening bleeding

Who gets FVIId?

- Children who inherit an abnormal gene from both parents. Men and women have an equal chance of being born with FVIId
- FVIId is rare, affecting an estimated 1 in every 300,000 to 500,000 people

What are the symptoms of FVIId?

The most common signs of FVIId, other than excessive bleeding after injury or invasive procedures, include:
- Frequent nosebleeds
- Bleeding from the gums
- Very heavy, prolonged menstrual bleeding
- Head bleeds in newborns
- Prolonged bleeding following circumcision
- Intestinal bleeding
FVIId can be detected at birth. However, some people with FVIId grow into adulthood without knowing they have it. The blood test that confirms congenital factor VII deficiency is called a clotting assay. It measures the amount of time it takes to create a clot.


Hear from people who
share your experience.
New to treatment? It’s
easy to get started.
![Icon: 5-step purification process of NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/cta/CTA-recombinant.jpg)
![NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/global/logo-novoseven-rt.png)

