In all bleeds in hemophilia A
and B with inhibitorsc
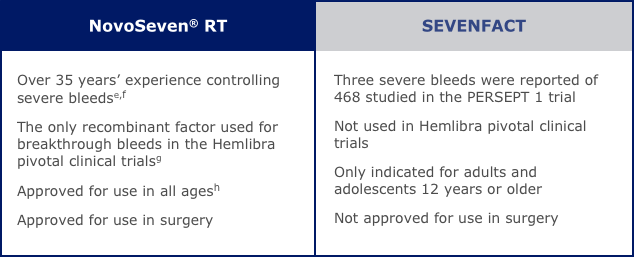
Effective treatment for hemophilia A with inhibitors.
NovoSeven® RT offers established bleed control that people with hemophilia A with inhibitors have trusted for more than 35 years.a It’s also the only rFVIIa that has published clinical experience for use with Hemlibra®.

Why NovoSeven® RT?
Why NovoSeven® RT?
aFor people with hemophilia A or B with inhibitors.
Get fast bleed control—even for the most life-threatening bleeds.b
A total of 227 bleeding episodes were studied. All bleeds refers to joint, target joint, skin, and mucous membrane, muscle, and other bleeding episodes.
In all severe
bleeding episodesd
A total of 518 severe bleeding episodes were studied. Bleeds included muscle, ENT (ear, nose, throat), CNS, joint, and internal/retroperitoneal bleeds.
In children in a trial with patients with hemophilia A and B with and without inhibitors
20 children younger than 12 and 8 children ages 12 to 16 were studied. Bleeds included joint, muscle, and mucous membrane bleeds.
bIn compassionate use situations including surgery, central nervous system hemorrhages, severe intra-abdominal bleeding, and other life-threatening bleeding episodes.
cProven effective in the adept™2 study, one of the largest clinical trials conducted in patients with hemophilia with inhibitors. The adept™2 study included people with hemophilia A with inhibitors (n=66) and people with hemophilia B with inhibitors (n=6). Of those who participated in the study, 57 were treated with NovoSeven® RT and 67 were treated with vatreptacog.
dData shown from compassionate use program and 2 clinical studies including patients with hemophilia A or B with or without inhibitors and acquired hemophilia.
Stay prepared for bleeds
Did you know that identifying bleeds quickly is important? Learn the signs and symptoms of bleeds so you can identify them when and where they happen.

NovoSeven® RT is the only rFVIIa used for breakthrough bleeds in the Hemlibra pivotal clinical trials. If you have hemophilia A with inhibitors and use Hemlibra, NovoSeven® RT can help you manage acute bleeding episodes that require a bypassing agent.
Established safety profile that comes from experience.e
In clinical trials of NovoSeven® RT, a low rate of blood clots (0.2% of bleeds) was reported in patients with congenital hemophilia.f It’s the first FDA-approved recombinant bypassing agent for hemophilia with inhibitors and is the only FDA-approved bypassing agent for all ages in people with hemophilia with inhibitors. The most common and serious side effects are blood clots.
The one and only NovoSeven® RT.

eCompassionate use, also known as expanded access, began enrolling in 1988; FDA approval received in 1999.
fFor people with hemophilia A or B with inhibitors.
gThe analysis included bleeding episodes in the HAVEN1, HAVEN2, and HAVEN4 clinical trials for which patients with CHAwI on emicizumab prophylaxis (at the labeled does) used rFVIIa. Initial individual dosing with rFVIIa, dosing intervals, and cumulative dosing were evaluated. All adverse events reported in each of the 3 trials, including available narratives, were assessed.
hIn people with acquired hemophilia, NovoSeven® RT is only indicated for adults.
Fast to infuse.
Faster infusion means NovoSeven® RT gets to work stopping bleeds more quickly.
NovoSeven® RT (90 mcg/kg)

FEIBA (75 U/kg)

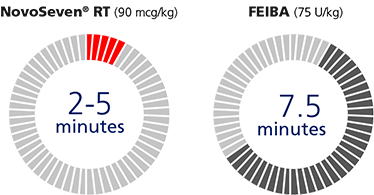
- Infusing takes 2 to 5 minutes
- NovoSeven® RT is faster to infuse than FEIBAi,j,k
Fast bleed control.
Helps control joint bleeds in as few as 5 hours with a median of 2 doses.i

- NovoSeven® RT can be redosed every 2 hours,l which lets you dose as needed to get bleeds under control fast
- Your hemophilia treatment center (HTC) and your doctor will help you decide which dosing schedule is best for you
iAdminister as a slow bolus injection over 2 to 5 minutes, depending on the dose administered.
jIndividual doses are compared and based on an 88-kg (194-lb) person.
kPatients are cautioned that the maximum injection or infusion rate must not exceed 10 units per kilogram of body weight per minute.
lBased on individual dosage for joint bleeds in people with hemophilia A or B with and without inhibitors.
Justin's story.
Justin lives with hemophilia A with an inhibitor. Learn about his love for travel and how NovoSeven® RT fits into his life when he’s on the go.
Room-temperature stable and travel ready.
NovoSeven® RT comes in compact packaging, and is room-temperature stable up to 77 °F, so you can be on the go.l
lNovoSeven® RT should be stored between 36 °F and 77 °F.
For complete storage information, please see Prescribing Information.

Quick to mix.m
NovoSeven® RT with MixPro® makes mixing a dose fast. The prefilled syringe means no extra steps to fill a syringe with diluent. See how to prepare a dose with MixPro® in our video.
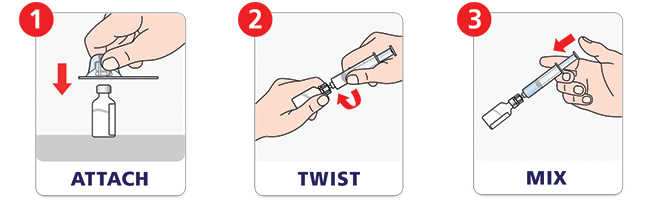
After mixing, NovoSeven® RT may be stored at room temperature in the vial for up to 3 hours.n
mCompared with mixing using histidine vials.
nFor complete storage and handling instructions, please see Prescribing Information.
What if I need surgery or a procedure?
We understand that you have concerns about controlling bleeds if you ever need surgery or a procedure. The good news is that NovoSeven® RT is approved by the FDA for treating people with hemophilia A or B with inhibitors during and after surgery and procedures.
In fact, in a clinical trial, NovoSeven® RT was proven to effectively control bleeds throughout the surgical process. Here are the results:
Percentage of patients with satisfactory bleed control:
The postoperative period: essential to surgical success
Talk with your doctor for more information on controlling bleeds during and after surgery or a procedure.
Why NovoSeven® RT
Explore the features of NovoSeven® RT and learn how it could fit your life. See the features
About hemophilia A with inhibitors.

What is hemophilia A?

Hemophilia is a bleeding disorder that prevents blood from clotting properly. There are a number of proteins in blood, called “clotting factors,” that work together in a series of steps to form blood clots. People with hemophilia A are missing factor VIII. Hemophilia mostly affects men, but it can also affect women. And although many people inherit the condition, about 1/3 of people with hemophilia don’t have a family history of it.

What are inhibitors?

Inhibitors are a complication of hemophilia. People with severe hemophilia A or B are usually treated by replacing the missing factor VIII or factor IX through infusion. For some people, however, this treatment does not work. Their bodies react as though the treatment is an invader and their immune system develops antibodies, or “inhibitors,” that attack and neutralize the factor VIII or IX. When this happens, the factor is not able to stop the bleeding.


Hear from people who
share your experience.
New to treatment? It’s
easy to get started.
![Syringe and vials of NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/cta/CTA-considering-novoseven.jpg)
![NovoSeven® RT (Coagulation Factor VIIa [Recombinant])](/content/dam/novonordisk/novoseven/global/logo-novoseven-rt.png)
